Lead acid batteries were the first form of rechargeable battery which are still used today. As we learned in history of batteries; lead acid batteries were developed in 1859 by Gaston Plante. This battery type is still used today in many industries, especially popular as car batteries, but also commonly used in photovoltaic systems.
Compared to other battery types, advantages of lead acid battery are that they are long lasting, and have low costs. However, they lack in energy density, have a moderate efficiency, and they have high maintenance demands.
Although they have low energy density, their power-to-weight ratio is relatively large which helps them supply high inrush currents.
Due to these low costs, and ability to supply high surge currents, they are most commonly used in car batteries to provide the starter motors with the current they require.
Functioning of Lead Acid Batteries
A lead acid battery consists of three main elements:
- Negative electrode made of spongy/porous lead (Pb)
- Positive electrode made of lead peroxide (PbO2)
- Electrolytic solution of dilute sulfuric acid (H2SO4) – ratio of water to acid 3:1
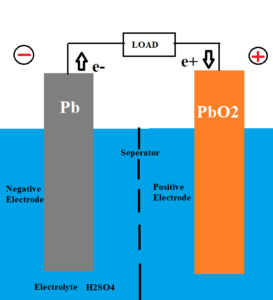
The basic arrangement of lead acid battery is done in the way shown in the image. Sponge lead plate and lead peroxide plate is dipped in electrolytic solution of dilute sulfuric acid.
To prevent the electrodes from coming into contact with each other, a chemically permeable but electrically insulating membrane acts as a seperator.
A load is externally connected between the two electrodes.
The molecules in dilute sulfuric acid (H2SO4) split into positive hydrogen ions (H+) and negative sulfate ions (SO₄²-).
When the hydrogen ions reach the positive electrode plate of PbO2 they receive electrons and become hydrogen atoms.
These attack PbO2 again and form PbO and H2O (water).
The PbO then reacts with H2SO4 and forms PbSO4 and H2O.
The overall chemical reaction is:
PbO2 + Pb + 2H2SO4 ←charge/discharge→ 2PbSO4 + 2H2O Eocell = 2.05 V
At negative terminal the reactions are:
Pb + HSO4– ←charge/discharge → PbSO4 + H++2e–
At positive terminal the reactions are:
PbO2 + HSO4– + 3H+ + 2e– ←charge/discharge → PbSO4 + 2H2ss
Charging and Discharging of Lead Acid Battery
Discharging
Both positive and negative plates become lead (II) sulfate (PbSO4) when the battery discharges.
The electrolyte becomes less concentrated due to losing sulfate from the dissolved sulfuric acid, essentially becoming water.
Charging
When the lead acid battery is fully charged, the negative plate comprises of lead, and the positive plate is lead oxide.
The electrolyte solution contains a higher concentration of sulfuric acid, which is what stores the chemical energy.
Overcharging
If the battery is overcharged by using high charging voltages, oxygen and hydrogen gasses are generated due to water being electrolyzed from the electrolyte.
This process is known as “gassing” of battery.
There are three main issues that arise due to gassing.
1) Safety hazard due to hydrogen gas being produced which is explosive
2) Water is bubbled out and lost from the battery, which must be replaced manually
3) Permanently reduced battery capacity due to active material being lost from the electrolyte
Lead Acid Battery Hazards and Safety
There are three potential hazards associated with lead acid batteries:
The electrolyte of the battery contains sulfuric acid, which is corrosive. To prevent any harm, it is recommended to wear protective clothing when working with batteries. Eye and foot protection should also be done.
As explained previously, there is a potential risk of explosion during charging, especially overcharging, as hydrogen and oxygen gasses are produced. In order to reduce risk of explosion, proper ventilation must be done as well as any sources of ignition (e.g., sparks from circuit) must be eliminated. Furthermore, avoid exposing a lead acid battery to direct sunlight as the plastic housing can become brittle.
Lastly, batteries generate high currents and if conductive materials are placed across the terminals current can flow through them. In order to avoid harm, prevent metallic/conductive objects (e.g., jewellery) to come into contact with the battery. Tools should have insulated handles to prevent shock.
Lead Acid Battery Maintenance
All sorts of lead acid batteries require some form of periodic maintenance in order to be in perfect working condition.
The basic and essential maintenance checks that should be done need to ensure three things.
The battery should always be checked to ensure that the external terminals are not corroded. The corrosion can be reduced by coating terminal with petroleum jelly or a product made to prevent corrosion.
It should be ensured that the connections to the battery are tight in order to avoid unnecessary problems.
Lead acid batteries are usually in plastic housing which should be free of any damage, cracks, or corrosion.
Apart from this, batteries also require refilling water which is lost, especially if a battery is overcharged.
Although this maintenance seems easy, the cost can be high due to the fact that if the batteries are at a remote location, someone has to periodically travel to the location to perform all the checks.

