Although magnesium battery is becoming less popular as compared to lithium batteries, still we need to understand the usage of magnesium batteries.
In these type of batteries, anode is made up of magnesium because of its high standard potential. Magnesium is a light metal, easily available and having low cost.
Magnesium/manganese dioxide (Mg/MnO2) battery has twice the service life i.e. as compared to capacity of the zinc/manganese dioxide (Zn/MnO2) battery of same size.
It has also the ability to retain its capacity, during storage, even at high temperatures.
Magnesium battery is durable since it has always a protective cover which is naturally formed on the surface of the magnesium anode.
The magnesium battery generally loses its capacity of storage once it has been partially discharged and that is why it is not very suitable for using in long-term intermittent applications.
This is the main reason, why magnesium battery is losing its popularity and lithium battery are occupying its market.
There is big potential to develop new magnesium-sulfur batteries for electric cars.
These batteries have the capacity to hold twice as much power as the best lithium-ion battery cells.
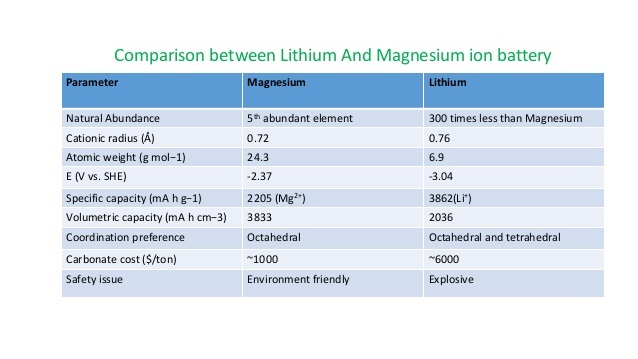
Below table shows a glimpse of the features both batteries are having.
Magnesium Battery Composition
In magnesium primary battery, magnesium alloy is used as anode; manganese dioxide is used as cathode material.
Since the manganese dioxide alone cannot provide required conductivity to the cathode, therefore acetylene black is mixed with manganese dioxide to achieve required conductivity.
Mainly magnesium per-chlorate is used as electrolyte and barium and lithium chromate are added to electrolyte for preventing corrosion.
Magnesium hydroxide is also added to this mixture as buffering agent to improve the ability of battery storage.
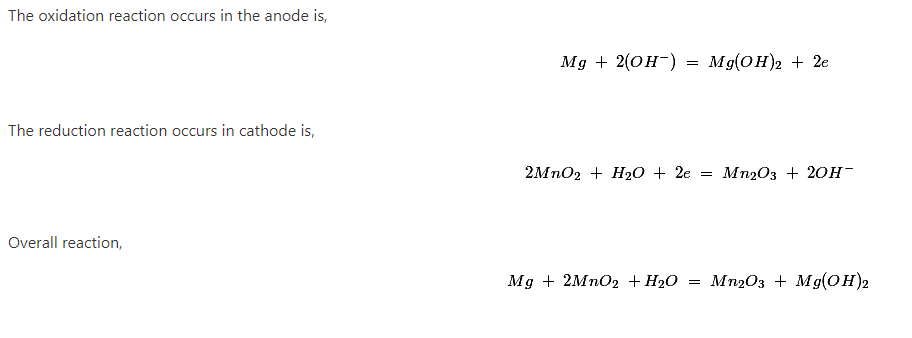
This cell gives open circuit voltage around 2 volt but the theoretical value of the cell potential is 2.8 volt.
The chance of corrosion of magnesium is very less even under extreme environmental conditions.
Actually raw magnesium reacts with moisture and forms a coating of thin film of Mg(OH)2 on its surface.
This thin film of magnesium peroxide serves as a corrosion protective layer over the magnesium.
In addition to that chromate treatment on magnesium improves this protection to very large extent.
But when this protective film of magnesium peroxide is puncher or removed due to discharge of battery , corrosion takes place with formation of hydrogen gas.
Construction of Magnesium Battery
Construction wise a cylindrical magnesium battery cell is similar to a cylindrical zinc carbon battery cell.
The main contained of the battery is made of an alloy of magnesium with small quantity of aluminum and zinc.
The cathode is of manganese dioxide. As the manganese dioxide has poor conductivity, acetylene black is mixed with this to improve its conductivity. This also helps to retain water inside cathode. In this cathode mixture barium chromate is added as an inhibitor, and also magnesium hydroxide is added as a pH buffer.
Magnesium per-chlorate with lithium chromate mixed in water is used as electrolyte.
A carbon is inserted in the cathode mix as current collector. Kraft papers, absorbed with electrolyte solution are placed in between cathode and anode materials as separators. Special attention is to be given during designing of sealing arrangement in magnesium battery.
It is important that the sealing of the battery is not so porous that the moisture inside the battery evaporates during storage of the battery. Also it should not be so nonporous that the hydrogen gas formed during discharge cannot escape from the battery.
Therefore the seal of the battery is formed in a way that it retains the moisture inside and at the same time it gives sufficient vent to the hydrogen gas formed during discharge. This is achievable by providing a small hole on the top of the plastic seal washer under the Retainer ring.
When excess gas comes out from the hole this retaining ring deforms due to pressure and helps escaping of the gas.
As compared to the generally magnesium anode another construction of magnesium battery is also available where carbon forms the outer container of the battery.
In this case a typical shaped container is formed from highly conductive carbon.
This container is formed in a cylindrical cup shape and one rod like shape is projected from its center as shown in the picture.
Anode of the battery is formed by a cylinder or drum of magnesium.
The diameter of the anode cylindrical is about half of the carbon cup.
The cathode mix is placed inside this anode cylinder and separated from the inner wall of the cylinder by a paper separator.
The space between inner surface of the carbon cup and outer surface of the anode cylinder also filled with cathode mix and here also the outer surface of the anode cylinder is separated from cathode mix by a paper separator.
The cathode mix is produced by mixing manganese dioxide, carbon black, and small quantity of aqueous magnesium bromide or per-chlorate as the electrolyte. Positive terminal is connected to the end of the carbon cup. The negative terminal is connected to the end of anode drum. The entire system is encapsulated in a crimped tin-plated steel jacket.
Advantage of Magnesium Battery
- Very good shelf life; it can be stored for long even under high-temperature. These battery can be stored up to 5 years at the temperature 20°C.
- Twice capacity compared to equivalent size Leclanche battery .
- Higher battery voltage than zinc-carbon battery .
- Cost effective
Disadvantages of Magnesium Battery
- Delayed action.(voltage delay)
- Evolution of hydrogen during discharge.
- Heat generated during use.
- Poor storage after partial discharge.
Sizes And Types Of Mg/Mno2 batteries
| BATTERY TYPE | DIAMETER IN MM | HEIGHT IN MM | WEIGHT IN GM | CAPACITY IN AH |
|---|---|---|---|---|
| N | 11 | 31 | 5 | 0.5 |
| B | 19.2 | 53 | 26.5 | 2 |
| C | 25.4 | 49.7 | 45 | 3 |
| 1LM | 22.8 | 84.2 | 59 | 4.5 |
| D | 33.6 | 60.5 | 105 | 7 |
| FD | 41.7 | 49.1 | 125 | 8 |
| No. 6 | 63.5 | 159 | 1000 | 65 |
Discover more from Electrical Engineering 123
Subscribe to get the latest posts sent to your email.
