If we are willing to understand the basic principle of battery properly, first, we should have some basic concept of electrolytes and electron affinity. Actually, when two dissimilar metals or metallic compounds are immersed in an electrolyte, there will be a potential difference produced between these metals or metallic compounds. Hence causing the flow of current which is due to potential difference in fact.
It is found that, when some specific compounds are added to water, they get dissolved and produce negative and positive ions. This type of compound is called an electrolyte. The popular examples of electrolytes are almost all kinds of salts, acids, and bases etc.
The energy released during accepting an electron by a neutral atom is known as electron affinity. As the atomic structure for different materials are different, the electron affinity of different materials will differ. If two different kinds of metals or metallic compounds are immersed in the same electrolyte solution, one of them will gain electrons and the other will release electrons.
Which metal (or metallic compound) will gain electrons and which will lose them depends upon the electron affinities of these metals or metallic compounds. The metal with low electron affinity will gain electrons from the negative ions of the electrolyte solution.
Electron Affinity in Battery Function
On the other hand, the metal with high electron affinity will release electrons and these electrons come out into the electrolyte solution and are added to the positive ions of the solution. In this way, one of these metals or compounds gains electrons and another one loses electrons. As a result, there will be a difference in electron concentration between these two metals. This difference of electron concentration causes an electrical potential difference to develop between the metals. This electrical potential difference or emf can be utilized as a source of voltage in any electronics or electrical circuit. This is a general and basic principle of battery.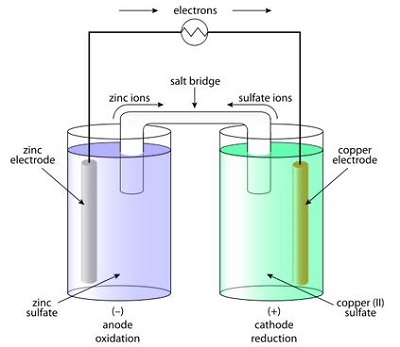
All battery cells are based only on this basic principle. As we know from battery history, Alessandro Volta developed the first battery cell, and this cell is popularly known as the simple voltaic cell. This type of simple cell can be created very easily. Take one container and fill it with diluted sulfuric acid as the electrolyte. Now immerse zinc and one copper rod in the solution and connect them externally by an electric load. Now your simple voltaic cell is completed. Current will start flowing through the external load.
Zinc in diluted sulfuric acid gives up electrons as below:



Daniell Battery Cell
The Daniell cell consists of a copper vessel containing copper sulfate solution. The copper vessel itself acts as the positive electrode. A porous pot containing diluted sulfuric acid is placed in the copper vessel. An amalgamated zinc rod dipping inside the sulfuric acid acts as the negative electrode.
When the circuit is completed, diluted sulfuric acid in the porous pot reacts with zinc so as to liberate hydrogen gas. The reaction takes place as below:


Discover more from Electrical Engineering 123
Subscribe to get the latest posts sent to your email.
