In order to understand the electric charge of electron let us first understand from where the charge comes? As all of us know that matter is made up of atoms. An atom consists of Proton, Neutrons and Electrons.
Proton is having the positive charge, electron is having negative charge and neutron is neutral means having no charge. Further protons and neutrons are inside the nucleus of an atom i.e. in middle and electrons are revolving around the nucleus.
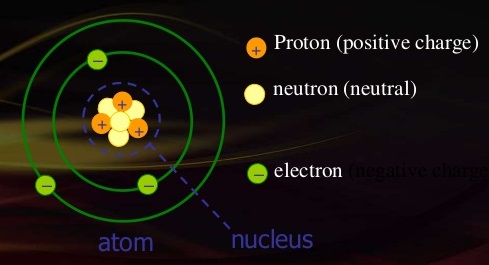
If in an atom electrons are equal to protons it is in neutral state, but if we remove some electrons from shells of an atom so it becomes positively charged because charge of proton becomes more. Inversely if we are able to add some electrons in an atom electrons become dominating hence atom is having negative charge due to excessive charge of electron.
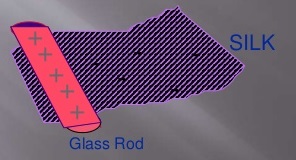
This is property of the charges that similar charges repel and opposite charges attract each other. To understand this just do a small experiment and rub a glass rod with silky amber cloth this causes transfer of electrons from cloth to rod and they start attracting each other now if we rub two pieces of cloths separately and bring them near to each other they will repel each other. This type of charge is known as static charge. The simplest form of electricity is called Static Electricity.
This universal principal of charges is the main reason of current flow. Lets understand from below example of battery which is always having positive and negative terminals. When we connect both terminals the electric charge starts flowing from negative to positive side.
Electric Charge Of Electron in Batteries
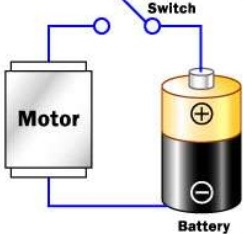
A basic law of the universe is that like charges repel and unlike attract. Two negatives will repel each other. A negative and a positive will attract each other. An electron has a negative charge. The negative terminal of a battery will push negative electrons along a wire. The positive terminal of a battery will attract negative electrons along a wire. Electric current will therefore flow from the negative terminal of a battery, through the lamp, to the positive terminal.
Due to the attractive forces negatively charged electrons are bound in their orbits by their attraction to the positive charge of protons. Any materials where these forces are weak it is easy to remove the electrons.
When an atom loses electrons it becomes a positively charged Ion. When an atom gains electrons it becomes a negatively charged Ion. The process by which atoms gain or lose electrons is called Ionization.
Some materials hold their electrons very tightly, therefore electrons do not move through them very well or may need extra force. These materials are known as insulators. Plastic, cloth, glass and dry air are good insulators. On the other hand materials like aluminum, gold, silver and copper are having loosely held electrons, which move through them very easily. These are called conductors. Most metals are good conductors.
Electric Charge Of Electron & Electrostatic Induction
The production of a charge in an uncharged body by bringing a charged object close to it is known as electrostatic induction. The charges that appear on the uncharged materials are known as induced charges.
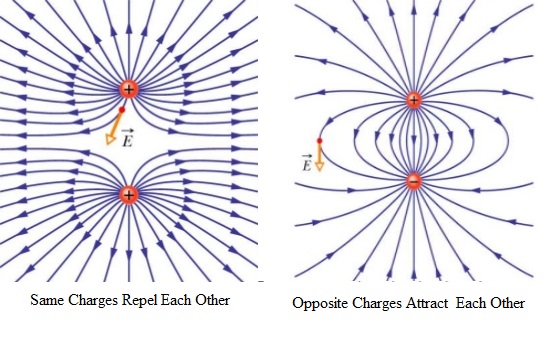
The region around the electric charges where another charge when placed will experience a force of attraction or repulsion is called an electric field. The direction of electric field is from the positive charge to the negative charge. Electric field is represented by imaginary lines called electric field lines.
The electric charge of an electron is very small and it is equal to 1.6 x 10-19 Coulomb. That means if we add the charge of 6.28 x 1019 electrons this will become equal to one Coulomb.
In other words if an object lacks 6.28 x 1019 number of electrons it will have 1 coulomb positive electric charge. Inversely the object having 6.28 x 1019 number of excess electrons it will have 1 coulomb negative electric charge.
Discover more from Electrical Engineering 123
Subscribe to get the latest posts sent to your email.

