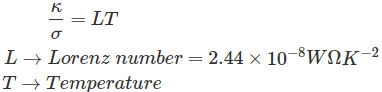The opposition to flow of electrons (due to bonds between protons and electrons, as well as to collisions) is called a electrical resistance (R). Or Resistance may also be defined as “The property of the electric circuit which opposes the flow of
Read More
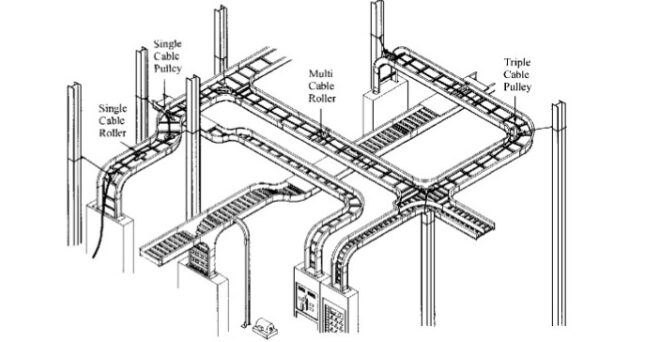
Cable tray / raceway is integral part of any cable management system. Selection of cable tray is very critical because if cable tray size is not sufficient the cables may become damaged due to improper handling and excessive heating etc. On the
Read More
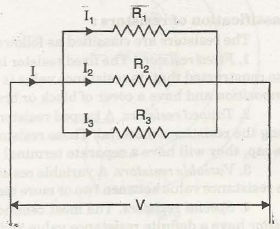
Kirchhoff’s Voltage Law states that net voltage gain and net voltage drops along a closed loop are equal. Kirchhoff’s Current Law states that at any point in the circuit the total current enters, is exactly equal to the total current leaves the point. There
Read More
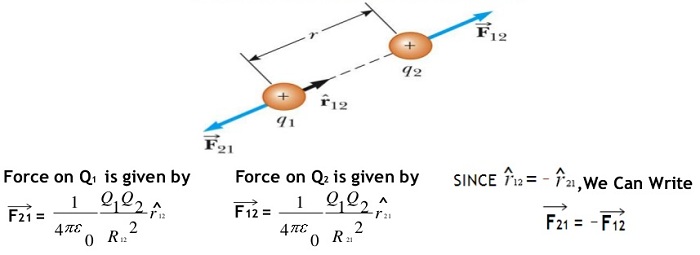
This page is dedicated for providing best and up to date information about the coulomb’s law. In addition you can find the definition, equation and formula for understanding the basic concept of coulomb’s law. You already know that when two charged bodies come closer
Read More
Conductivity refers to the ability to conduct, convey or pass through, and in case of electricity it refers to ability to convey the electric current. On the other hand for thermal conductivity it is the ability to conduct or pass the heat
Read More
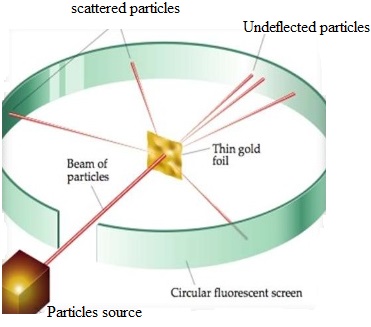
There are many names in the field of atomic model and atomic structure. The first name to understand the atomic structure of matter was J.J.Thomson who represented the Plum Pudding Model, that was based upon the concept that electrons in an atom are distributed
Read More